Jon D. Rainier
 ORGANIC CHEMISTRY
ORGANIC CHEMISTRY
Professor
B.S., University of California, Irvine, 1985
M.S., California State University, Long Beach, 1989
Ph.D., University of California, Riverside, 1993
National Institutes of Health Postdoctoral Fellow, University of Pennsylvania, 1993-96
Phone: 801-581-4954 (office), 801-581-4716 (lab)
Office: 3152 HEB
Email: rainier@chem.utah.edu
Research Group
Biological Chemistry Program
Activities & Awards
- University of Utah Distinguished Teaching Award, 2013
- ASUU Student Choice Teaching Award, 2011
- Gencorp Award for Excellence in Chemical Synthesis, 2000
- Research Corporation, Research Innovation Award, 1999
- National Science Foundation Career Award 1999-2003
- National Institutes of Health, First Award, 1998-2002
Research Interests
Our primary interest lies in the field of chemical synthesis. Within this discipline, we are particularly facinated by the interplay between structure (biological activity), total synthesis, and reaction development.
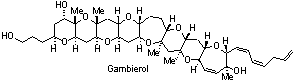
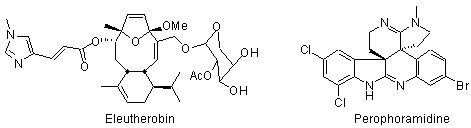
Total Synthesis and Structure. Biologically active targets that are currently receiving attention in our laboratory include: (a) fused polycyclic ether natural products (e.g. gambierol, gambieric acid, psymberin); (b) indoline and oxindole natural products (e.g. perophoramidine, communesin B); (c) oxygenated cembranoid natural products (eleutherobin, eunicellins, labiatins); (d) the general class of indolizidine and pyrrolizidine alkaloids; (e) others.
Reaction Development. We are equally involved in reaction development with a particular emphasis on the use of new and/or improved methods to the synthesis of the targets mentioned above. Reactions that are currently being examined in our laboratory include: (a) new methods to synthesize carbon-glycosides; (b) enol ether-olefin ring-closing metathesis reactions to fused polycyclic ethers; (c) the use of rhodium carbenoids to generate highly substituted indoles and indolines; (d) anionic fragmentation and condensation reactions of bicyclic ring systems; (e) tandem ring-opening/cross metathesis (ROCM) and ring-opening/ring-closing metathesis (RORCM) reactions of norbornenes.
Selected Publications
- Orendt, A. M.; Roberts, S. W.; Rainier, J. D. "The Role of Asynchronous Bond Formation in the Diastereoselective Epoxidation of Cyclic Enol Ethers: A Density Functional Theory Study," J. Org. Chem. 2006, 71, 5565.
- Sabahi, A.; Novikov, A.; Rainier, J. D. "2-Thioindoles as Precursors to Spiro-Fused Indolines: Synthesis of (±)-Dehaloperophoramidine," Angew. Chem. Int. Ed. 2006, 45, 4317.
- Majumder, U.; Cox, J. M.; Johnson, H. W. B.; Rainier, J. D. "The Total Synthesis of Gambierol. The Generation of the A-C and F-H Subunits Using a C-Glycoside Centered Strategy," Chem. Eur. J. 2006, 12, 1736.
- Johnson, H. W. B.; Majumder, U.; Rainier, J. D. "The Total Synthesis of Gambierol. Subunit Coupling and Completion," Chem. Eur. J. 2006, 12, 1747.
- Liu, Z.; Rainier, J. D. "Ring-Opening/ring-Closing Metathesis (RORCM) Reactions of 7-Azanorbornene Derivatives. An Entry into Perhydroindolines," Org. Lett. 2006, 8, 459.
- Majumder, U.; Rainier, J. D. "Olefinic-ester cyclizations using Takai-Utimoto reduced titanium alkylidenes," Tetrahedron Lett. 2005, 46, 7209.
- Roberts, S. W.; Rainier, J. D. Substitution and Remote Protecting Group Influence on the Oxidation/Addition of a-Substituted 1,2-Anhydroglycosides: A Novel Entry into C-Ketosides," Org. Lett. 2005, 7, 1141.
- Johnson, H. W. B.; Majumder, U.; Rainier, J. D.* "The Total Synthesis of Gambierol," J. Am. Chem. Soc. 2005, 127, 848.
- Liu, Z.; Rainier, J. D. "Regioselective Ring-Opening/Cross-Metathesis Reactions of Norbornene Derivatives with Electron Rich Olefins," Org. Lett. 2005, 7, 131.
- Nyong, A.; Rainier, J. D. "The diastereoselective synthesis of quaternary substituted thioindolines from sulfur ylide intermediates, J. Org. Chem. 2005, 70, 746.
