Aurora Clark
 PHYSICAL/MATERIALS/INORGANIC CHEMISTRY
PHYSICAL/MATERIALS/INORGANIC CHEMISTRY
Professor
Ph.D., Indiana University, 2003
Directors Postdoctoral Fellow, Los Alamos National Laboratory Theory Division 2003-2005
Professor, Department of Chemistry, Washington State University, 2005-2022
Phone: (801) 581-5896
Office: 4617 TBBC
Email: aurora.clark@utah.edu
Research Group
Activities & Awards
- Fellow, APS (2021); AAAS (2019); ACS (2017)
- WSU Young Faculty Performance Award, Mid-Career Award, Professional Service Award, Undergraduate Research Excellence Mentor Award
- Volunteer of the Year, Washington Idaho Border Section of the ACS
- ACS HP Junior Faculty Award
- ACS PROGRESS/Dreyfus Lectureship
- Editorial Board Journal of Physical Chemistry, Journal of Chemical Physics, Solvent Extraction and Ion Exchange, Computational and Theoretical Chemistry
- Chair, DOE Council on Chemical Sciences, Geosciences and Biosciences (2017 – 2021)
- NASEM Committee on a Research Agenda for a New Era in Separations Science
- Director, WSU Center for Institutional Research Computing (2016 – 2022), Materials Science and Engineering Program (2014 – 2017), WSU-PNNL Nuclear Science and Technology Institute (2016 – 2019)
Research Interests
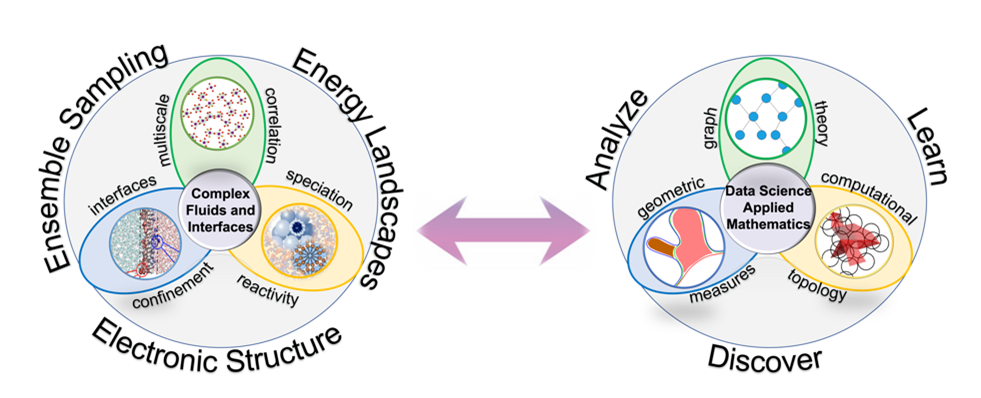
Our laboratory uses atomistic simulations (ab-initio and classical molecular dynamics) and data science to characterize the structure and reactivity of complex and chemically extreme solutions and their interfaces. We advance graph theory, topological data analyses, and machine learning to develop new models that link molecular-scale correlations and reactivity with micro- and mesoscale structure and dynamics.
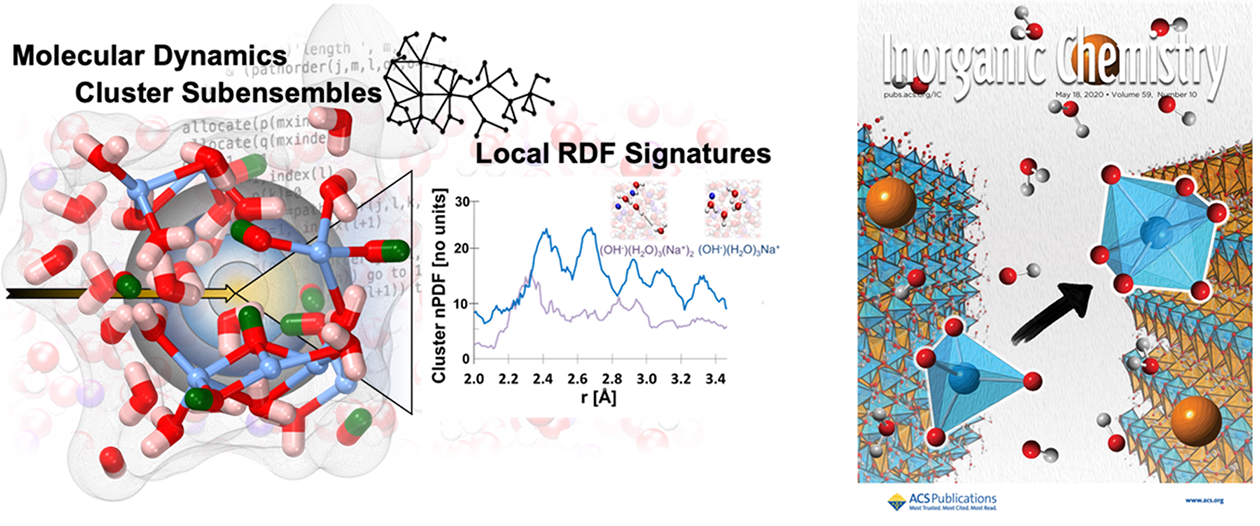
Chemistry that Improves Industrial Processing: Structure and Reactivity of Concentrated Electrolytes. Concentrated electrolytes permeate industrial processes that span the production of critical materials, separation and purification, environmental remediation, and in end-use technologies like batteries. Multiscale organization influences conductivity, rheology, and response to stimuli. This begins with local solvation, ion-ion interactions, and extends to percolated networks of ion interactions. As part of the DOE Energy Frontier Research Center on Interfacial Dynamics in Radioactive Environments and Materials (IDREAM) our team leads the computational studies of complex environments characterized by extremes in alkalinity and low-water activity to help accelerate the processing of legacy high-level radioactive waste and support energy efficient approaches for metal purification and processing. We work closely with collaborators at Pacific Northwest, Oak Ridge, and Argonne National Laboratories to predict and interpret spectroscopic and scattering profiles and elucidate properties and reactivity.
Basic Science to Support Environmental Cleanup and Human Health: Solution Interfacial Structure, Reactivity and Transport. Liquid interfaces exhibit unique reactivity, microenvironments and disorder due to the anisotropic character of the two phases. They underpin purification methods of drugs, contaminated materials, biological transport and catalytic transformations. The mechanisms of solute transport across a liquid/liquid phase boundary is an unsolved puzzle that limits the design of energy efficient and green separations technologies. Our laboratory has used molecular simulation and data science to identify interfacial reactions and new mechanisms of solute transport. Our lab has developed a classification system based upon solution conditions and interfacial organization, helping to resolve and interpret large variations in experimentally observed transport kinetics.
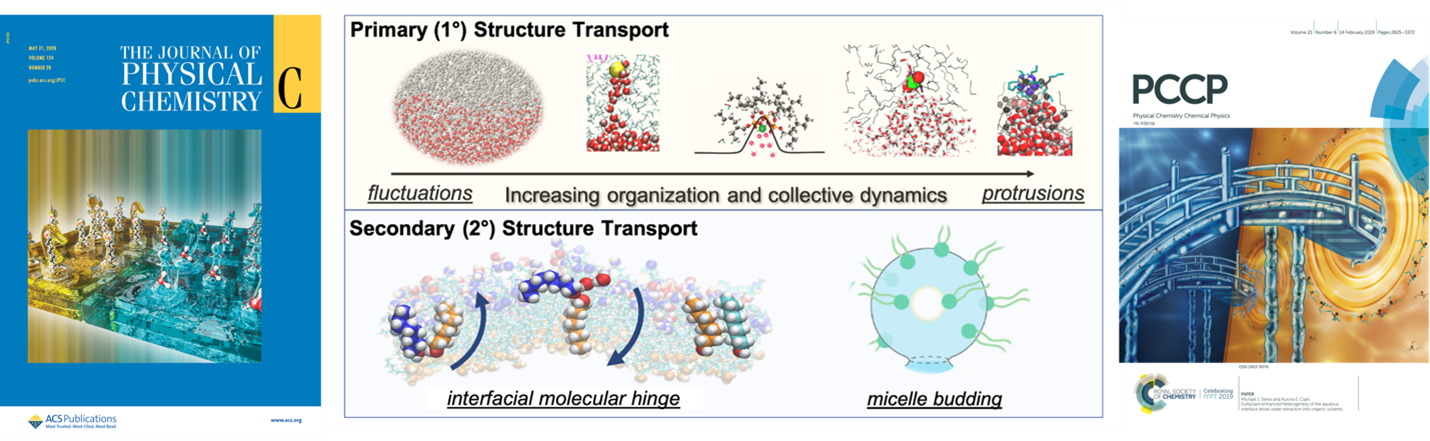
In our projects associated with solid/liquid interfaces, we seek to understand how the interfacial structure (water structure, solute concentration gradients and surface defects) impacts surface acid-base chemistry, adsorption phenomena and chemical transformations, and solid-state morphology. This allows us, for example, to predict preferred nanoparticle morphologies synthesized under different pH conditions and how to optimize nanoparticle morphology to enhance sorbent capacity of environmental contaminants for environmental cleanup.
Interdisciplinary Mathematics and Chemistry – Expanding the Paradigm of Data Science
in Chemistry. Although machine learning (regression) can help to predict behavior or properties
of chemical systems, a major challenge is to maintain interpretability so that we
can use the predicted correlations to improve or develop new chemical theories. We
apply and advance applied mathematics methods that retain chemical information as
we design new descriptors and perform machine learning to understand structure property
relationships across length and timescales (from electronic structure to molecular
organization and 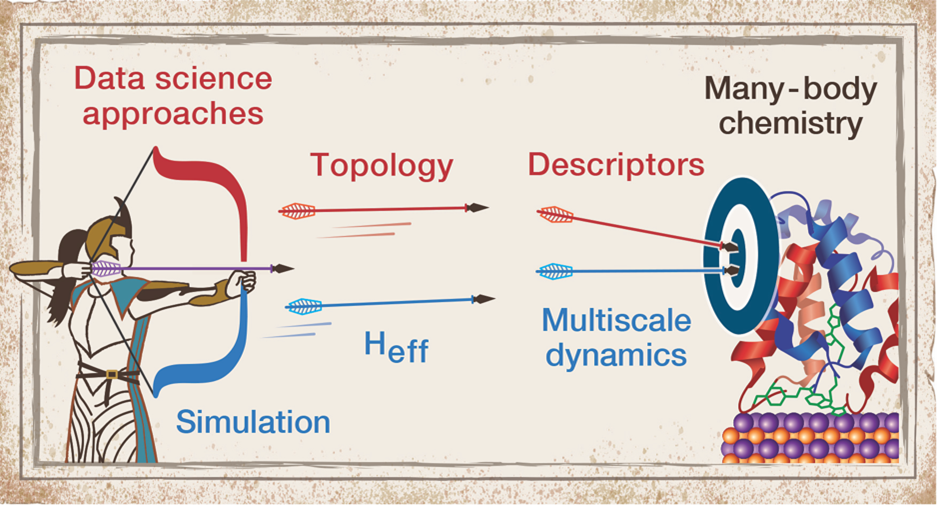 ensemble observations). Our laboratory is a pioneer of modern applications of graph
theory in chemistry, where we have focused upon networks of intermolecular interactions,
particle forces, etc and have advanced spectral graph theory descriptors, clustering methods and temporal graph theory analyses to characterize energy landscapes and chemical
transformations. Complementing our graph theory methods, we are developing topological
data analysis methods to encode topological information of energy landscapes so that we can mathematically combine information about structure and energy landscapes
of chemical systems. Augmenting these foundational methods are new techniques that
identify complex structures and shapes embedded within simulation data. Here geometric measure theory and persistent homology are actively employed to identify and characterize reactive,
transient and emergent structures in complex solutions and at interfaces that are
responsible for their unique chemical behavior.
ensemble observations). Our laboratory is a pioneer of modern applications of graph
theory in chemistry, where we have focused upon networks of intermolecular interactions,
particle forces, etc and have advanced spectral graph theory descriptors, clustering methods and temporal graph theory analyses to characterize energy landscapes and chemical
transformations. Complementing our graph theory methods, we are developing topological
data analysis methods to encode topological information of energy landscapes so that we can mathematically combine information about structure and energy landscapes
of chemical systems. Augmenting these foundational methods are new techniques that
identify complex structures and shapes embedded within simulation data. Here geometric measure theory and persistent homology are actively employed to identify and characterize reactive,
transient and emergent structures in complex solutions and at interfaces that are
responsible for their unique chemical behavior.
